
Stable quality and high purity achieved through all the manufacturing processes in Japan.
Sodium Hyaluronate for Pharmaceutical Use has complied with world pharmaceutical regulations.

Stable quality and high purity achieved through all the manufacturing processes in Japan.
Sodium Hyaluronate for Pharmaceutical Use has complied with world pharmaceutical regulations.


Our Sodium Hyaluronate is an extracellular polymeric substance derived from “Streptococcus zooepidemicus”, which is a kind of lactic acid bacteria, and is of non-animal origin.
Our Sodium Hyaluronate complies with world pharmaceutical regulations, and as an API supplier, is highly regarded in the European, US, Japanese, Korean, and Indian markets.
| Country/Region | Pharmaceutical registration/Manufacturing approval | Regulatory authority |
|---|---|---|
| EU | Certificate of Suitability to the European Pharmacopoeia | EDQM |
| US | Drug Master File for US FDA | FDA |
| Korea | Drug Master File | MFDS |
| India | API Registration Certificate | CDSCO |
| Japan |
Master file |
PMDA |
| European Pharmacopoeia | Sodium Hyaluronate |
|---|---|
| Japanese Pharmacopoeia | Purified Sodium Hyaluronate |

We offer Sodium Hyaluronate produced by microbial fermentation, which has been approved by the Japan Muslim Association (JMA) for HALAL certification (JAKIM, Malaysia) .
We have products of different molecular weights and viscosities from high to low molecular weight and even from high to low viscosity. This allows the products to be used in a wide range of pharmaceutical preparations (FDF).
|
Product Name |
SODIUM HYALURONATE | |||||
|---|---|---|---|---|---|---|
| Pharma Grade 80 | GS-40 | GS-100 | GS-120 | GS-200 | GS-300 | |
| Intrinsic Viscosity (m3/kg) |
1.55–2.00 | 0.39-0.99 | 1.00–2.49 | 1.5-2.0 | 2.50–3.31 | 3.32-4.60 |
| Shelf Life | 3 Years* | 2 Years* | 3 Years* | |||
| Storage Conditions | 2 to 8℃ | -25 to -15℃ | ||||
| Packaging | 100g | 100g, 200g | 100g | |||
| Bacterial Endotoxins (EU/g) |
Not more than 2.5** | Not more than 40** |
Less than 50** | Not more than 40** | Less than 50** | |
The above values are for reference only. For official standards, please request the specifications.
Compliance with MF and other pharmaceutical registrations varies from product to product.
GQP contracts are available within the scope as necessary.
* The shelf life is based on the normal manufacturing intrinsic viscosity(I.V.) of each grade.
** EP standard is less than 0.05 (EU/mg).
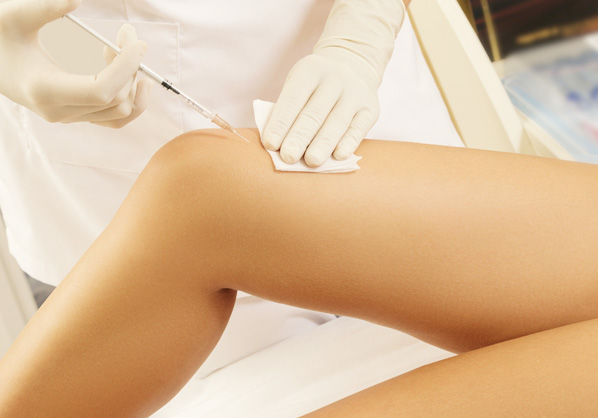
Joint injection
Knee injection (Osteoarthritis / Rheumatoid arthritis) / Bone regeneration
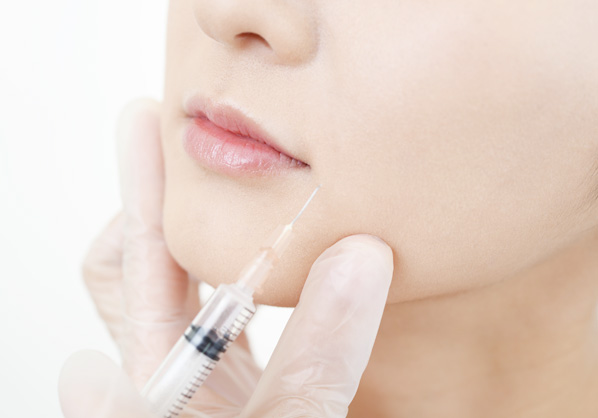
Cosmetic surgery
Dermal filler / Lip contouring
Body contouring
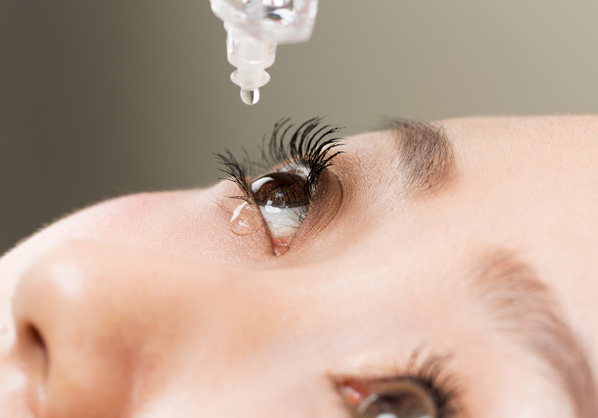
Ophthalmology
Ophthalmic Viscoelastic Device (OVD)
Eye drops / Contact lens solution
Intraocular lens insertion devices

External use
Skin preparation for external use
Topical skin application / Wound dressing
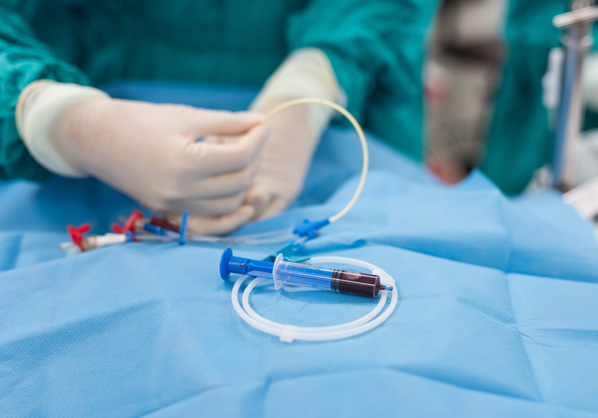
Medical device
Endoscopy, Catheter coating / Anti-adhesion
Vesicoureteral reflux treatment
Endoscopic mucosal resection (EMR)
Requests for information: If you are interested in our products, please feel free to contact us for further information, including catalogs, specifications, SDSs, and requests for samples.