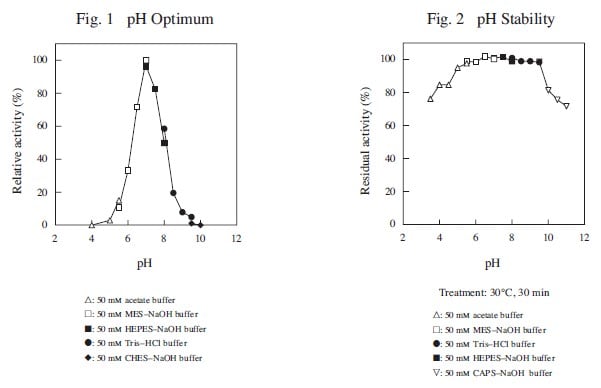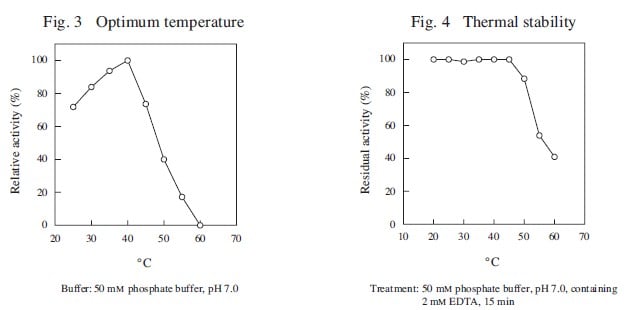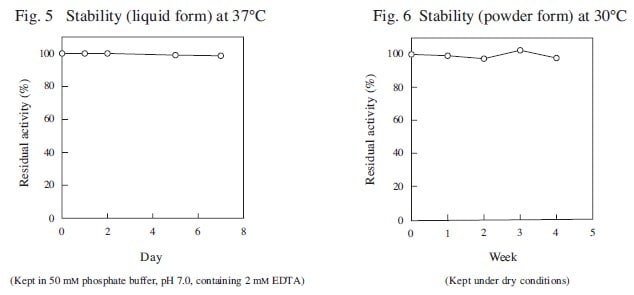
CD : 60189
The enzyme is useful for the determination of α-amylase and inorganic phosphate in clinical analysis.
| Origin | recombinant E. coli |
|---|---|
| Systematic name | β-D-Glucose 1,6-phosphomutase |
| EC Number | 5.4.2.6 |
| Reaction formula | β-D-Glucose 1-phosphate →→→ β-D-Glucose 6-phosphate |