Yeast and Mold Count (YMC) Testing
Yeast and Mold Count (YMC) testing is a widely used microbiological method for detecting and quantifying viable yeasts and molds in food, beverage, and environmental samples. These fungi are common spoilage organisms capable of thriving under challenging conditions such as low pH, reduced water activity, and refrigeration. As a result, YMC testing serves as a vital quality control tool for evaluating product integrity, sanitation effectiveness, and storage conditions. It is frequently applied in shelf-life determination and routine food safety monitoring.
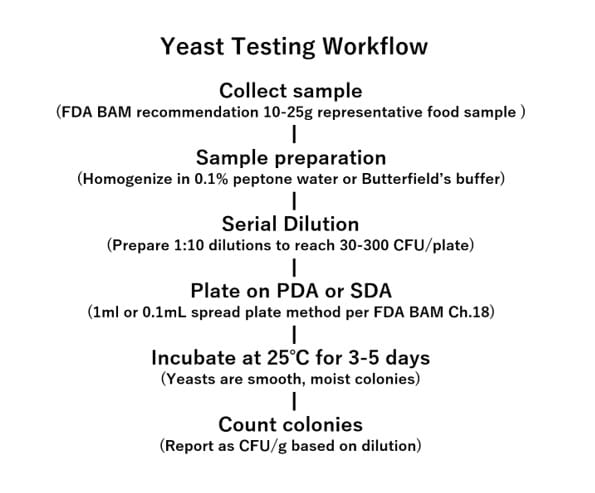
As outlined in the Food Sanitation Inspection Guidelines: Microbial Edition, Revised 2nd Edition (2018), standard YMC testing involves culturing samples on media like Potato Dextrose Agar (PDA) or Sabouraud Dextrose Agar (SDA), often supplemented with acid or antibiotics to suppress bacterial growth. Samples are incubated aerobically at 25℃ ± 1℃ for 5 to 7 days. Colonies—typically creamy for yeasts and fuzzy for molds—are then counted and reported as colony-forming units per gram (CFU/g) or per milliliter (CFU/mL).
While this method provides a general assessment of fungal contamination, it may not detect all organisms, especially slow-growing molds, heat-damaged yeasts, or those requiring specific growth conditions. In such cases, adjustments to media, temperature, or incubation duration may be necessary. For species identification beyond enumeration, further analysis such as microscopy or molecular testing is required.
Importance of Yeast and Mold Testing in Food Safety
Yeasts and molds are types of fungi commonly found in the environment, including in air, soil, and water. While certain yeasts play beneficial roles in food production (e.g., fermentation, baking, or brewing), uncontrolled fungal growth in food manufacturing settings can lead to product spoilage, discoloration, off-odors, texture changes, and potentially harmful mycotoxins.
Within Good Manufacturing Practices (GMP) and Hazard Analysis and Critical Control Points (HACCP) frameworks, YMC testing functions as a critical hygiene indicator. The resilience of yeasts and molds under low-moisture, acidic, and refrigerated conditions makes them particularly challenging in products such as dried foods, fermented items, and vacuum-packed goods.
The Food Sanitation Inspection Guidelines: Microbial Edition, Revised 2nd Edition (2018) provides standardized procedures for monitoring yeasts and molds in both products and processing environments.
Routine testing supports the following objectives:
- Early detection of microbial contamination
- Verification of cleaning and sanitation protocols
- Establishment of microbiological baselines
- Root cause analysis in spoilage investigations
- Shelf-life optimization and product stability
- Compliance with domestic and international food safety standards
Environmental monitoring, including air, surfaces, and equipment—alongside product testing plays a fundamental role in microbiological control. Adhering to standardized protocols ensures reliable results that support food safety risk assessments and prompt corrective actions.
Reference: Food Sanitation Inspection Guidelines: Microbial Edition, Revised 2nd Edition, 2018
Yeast and Mold Count Workflow
Workflow for Yeast Testing
Workflow for Mold Testing

Standards and References
- Food Sanitation Inspection Guidelines: Microbial Edition, Revised 2nd Edition, 2018
- ISO 21527-1:2008 – Microbiology of food and animal feeding stuffs — Enumeration of yeasts and molds
- FDA BAM Chapter 18: Yeasts, Molds, and Mycotoxins
Products for Aerobic Plate Counts
This is a microbial test film medium that does not require medium preparation and can be used immediately after opening.
Rapid (48 hour) detection of Yeast and Mold. The mold colonies spread widely and form purple colonies with diffuse edges. Yeast colonies form small circles and have defined edges.
Learn more about the product


