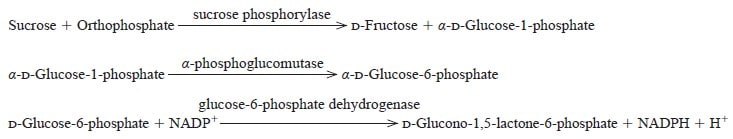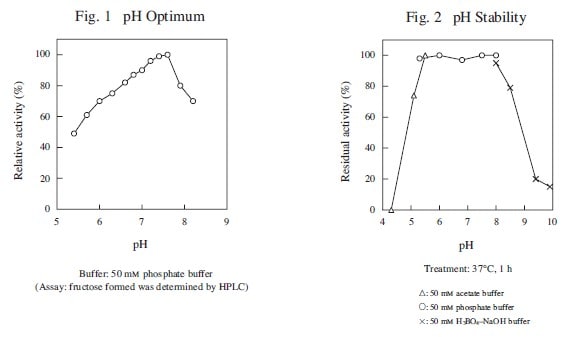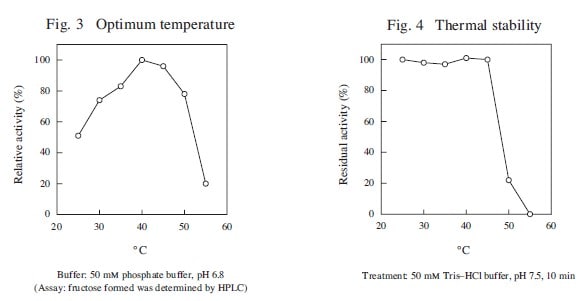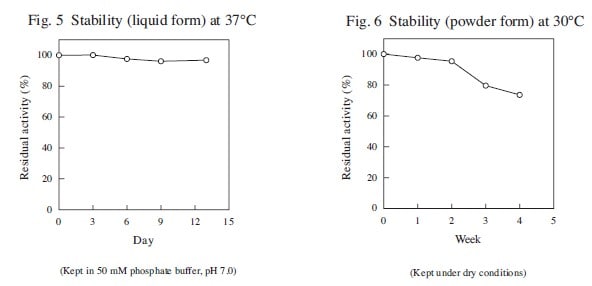
The enzyme is useful for the determination of inorganic phosphate in clinical analysis.
| 由来 | recombinant E. coli |
|---|---|
| 系统名称 | Sucrose : orthophosphate α-D-glucosyltransferase |
| EC编号 | 2.4.1.7 |
| 反应式 | Sucrose + Orthophosphate →→→ D-Fructose + α-D-Glucose 1-phosphate |